Abstract
Introduction: CD19-directed chimeric antigen receptor T (CART19) cell therapies have shown impressive clinical outcomes in CD19+ B-cell malignancies. All the FDA-approved CART19, including tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, and lisocabtagene maraucel use an anti-CD19 single-chain variable fragments (scFv) derived from the FMC63 antibody that binds to a CD19 epitope that is located in the membrane-distal portion of CD19. While this CART19 products are very effective in the clinic, the majority of patients still fails treatment or eventually relapses due to several mechanisms of resistance, including CAR T cell dysfunction in the immunosuppressive microenvironment. Novel strategies to enhance the activity of CART cells are critically needed. We and others recently demonstrated that modifications of the binding region of the CAR (scFv) (Singh N., Nat Med, 2021) can drastically change the interaction between the CAR T cell and the cancer cells, potentially improving the anti-tumor effect. In this study, we aimed to develop a novel anti-CD19 CAR that binds to a membrane-proximal domain of CD19 with the goal of improving CART functions. Moreover, we hypothesized that such a CART product would be active against B-cell acute lymphoblastic leukemia (B-ALL) blasts that present aberrant expression of the CAR19(FMC63) on the surface as we described in two pediatric B-ALL patients relapsed after CART19 (CTL019) at our Institution (Ruella M., Nat Med, 2018).
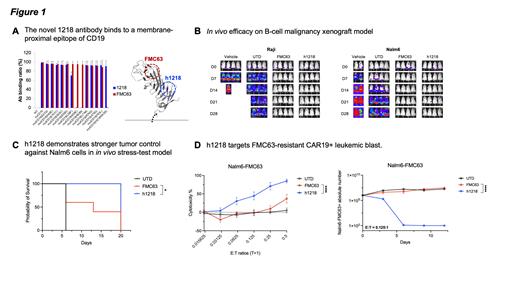
Methods and Results: To these goals, we screened a chicken immune scFv library against the CD19 extracellular domain. We selected an scFv clone (1218) that was not competing with FMC63 for CD19 binding in competition enzyme immunoassays and real-time interaction analysis. The chicken 1218 scFv was humanized by CDR-grafting to human germline genes and backmutations (h1218 scFv) and was confirmed to be specific for CD19 in cell microarray assays and flow cytometry analyses. In order to define the epitope that was recognized by the 1218 scFv, we performed mutagenesis assays replacing several residues of human CD19 with monkey CD19 residues showed that, as compared to FMC63, the epitope of the h1218 scFv is localized in a membrane-proximal location shown by three-dimensional modeling (Teplyakov A., Proteins, 2018) (Fig. 1A). Key residues needed for the binding of the h1218 scFv were identified as T51, S53, E55, K59 and K63, while the membrane-distal amino acid H218 is recognized as a key residue for FMC63 binding.
Therefore, we designed a novel CAR construct using the h1218 scFv, a 4-1BB costimulatory and CD3z signaling domains, using a lentiviral backbone. This CAR(h1218) construct was very active in vitro in human T cells demonstrating cytotoxicity, cytokine release, and proliferation upon encounter with CD19+ leukemic and lymphoma cells (NALM6, RAJI, and OCI-Ly18) (data not shown). Moreover, CART19(h1218) showed enhanced long-term (12 days) tumor control as compared to CART19(FMC63) against wild type NALM6 (data not shown). In vivo, CART19(h1218) cells (1.5x10 6 CAR+ cells/mouse) demonstrated tumor clearance against both Raji (CD19+ B-cell lymphoma) and Nalm6 (CD19+ B-cell leukemia) xenografts (Fig. 1B). Remarkably, in a more challenging stress- test model with lower CART doses (0.75x10 6 CAR+ cells/mouse) CART19(h1218) demonstrated stronger tumor control against Nalm6 cells as compared to CART19(FMC63) (Fig. 1C). Furthermore, our group recently described the occurrence of a B-ALL relapse characterized by the expression of the CAR19 itself on the cell surface due to accidental transduction of leukemic cells during manufacturing. The CART19(h1218) product but not CART19(FMC63) was able to recognize and kill CAR19(FMC63)-expressing leukemic cells in vitro at varying E:T ratios. Of note, in a long-term (12 days) in vitro killing assay using a low, challenging E:T ratio of 0.125:1 we observed potent and prolonged tumor control (Fig. 1D).
Conclusions: These results indicate that the development of a CAR binder able to recognize an alternative and membrane-proximal epitope of CD19 might lead to improved anti-leukemia and lymphoma activity and can recognize CAR19(FMC63)+ blasts that are not recognized by CART19(FMC63). This novel CART19(h1218) product will be tested in phase I-II clinical trials for patients with B-cell malignancies.
Patel: AbClon Inc.: Research Funding. Kim: Abclon Inc.: Current Employment. Lee: AbClon Inc.: Research Funding. Guruprasad: AbClon Inc.: Research Funding. Kim: AbClon Inc.: Current Employment. Choi: AbClon Inc.: Current Employment. Kim: AbClon Inc.: Current Employment. Kim: AbClon Inc.: Current Employment. Lee: AbClon Inc.: Current Employment. Lee: AbClon Inc.: Current Employment. Park: AbClon Inc.: Current Employment. Lee: AbClon Inc.: Current Employment. Cui: AbClon Inc.: Current Employment. Yoon: AbClon Inc.: Current Employment. Kim: AbClon Inc.: Research Funding. Kim: AbClon Inc.: Research Funding. Hwang: AbClon Inc.: Current Employment. Lee: AbClon Inc.: Current Employment. Lee: AbClon Inc.: Current Employment. Chung: AbClon Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ruella: Novartis: Patents & Royalties; BMS, BAYER, GSK: Consultancy; AbClon: Consultancy, Research Funding; Tmunity: Patents & Royalties; viTToria biotherapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal